General Requirements
The surgery room, where the surgery and procurement are carried out, is generally built to keep biocontamination to a minimum. It is therefore important to do as little as possible to risk the contamination of the tissue and to utilise strictly sterile material.
During the surgery, the surgeon must evaluate the physical quality of the femoral head, and then fill in a Procurement Report, a copy of which will need to be sent to us. The scrub nurse has the task to take care of the packaging.
The entire surgical team must work to keep the contamination risk to a minimum, including any unnecessary handling of the femoral head.
The scrub nurse can carry out the sampling for the microbiological screening, with the kit provided by their laboratory or by us, upon request. In this case, the femoral head must be sent directly to us, for microbiological testing.
Sampling and Packaging of the Femoral Head
To open and use the packaging kit, see our operative photographic instructions “Opening and use of the kit” Mod 79 SL.
For the microbiological sampling and how to package the femoral head see our operative photographic instructions “Sampling and packaging of the femoral head” Mod 78 SL.
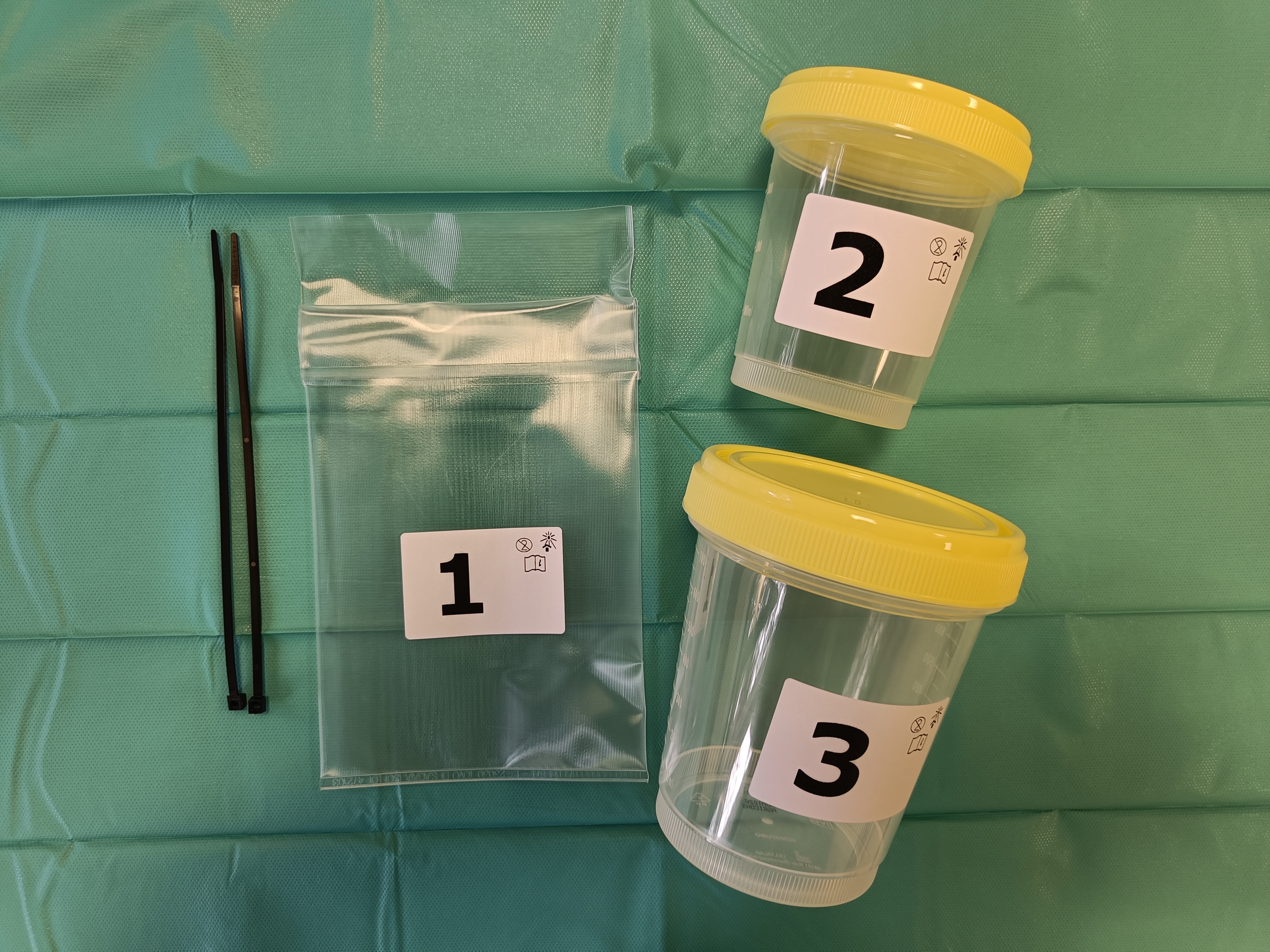
Microbiological Screening
The microbiological screening is carried out to detect the presence of aerobic or anaerobic bacteria and fungi. Microbial growth detection must be carried out via the use of culture medium. The incubation of the samples must not be under 7 days. Subcultural modality must be also carried out to isolate and identify microorganisms.
Storage
After removal, the tissue must be frozen as quickly as possible, at -80°C in a freezer with an adequate monitoring and alarm system. The tissue can also be kept at a temperature below +10°C until transported to the ultimate storage place/facility. However, it must be frozen at -80°C within 12 hours from the donation. Please keep the package ready to send in a separate freezer possibly with restricted access.
Shipment details
The shipment must be done within a month from procurement, satisfying the following requirements:
- The tissue must be correctly packaged and labelled
- The plasma must be aliquoted and labelled
- You must send a copy of the donor clinical reports, certifying preliminary suitability, carried out in your facility
- You must send a copy of the cultural screening (if carried out in your facility)
- The documentation must be thoroughly filled in (see our reserved area):
- Report of procured femoral heads
- M02-PO-01 - Donor Data Collection
- Mod 97 sl – Informed consent
- Mod 72 sl – Preliminary Donor Suitability
- Mod 3 sl – Viral diseases form
Transportation
For transportation of plasma samples and femoral heads, you must use insulating containers filled with dry ice or ice bricks, enough to cover the journey from your facility to ours. You must organise transportation so that the cold chain is maintained at all times. If your facility is very distant from us, we recommend you the use of dry ice.
It is recommended that you send the shipment as soon as possible after the screenings required. In any case, the delivery must be done within a month from the procurement of the tissue.
You must also deliver the following documentation, thoroughly filled in and signed:
- Laboratory reports (Viral screening, SARS CoV-2 PCR test)
- A copy of the Procurement Report of the musculoskeletal tissue (if provided by your facility), where the surgeon has specified physical and morphological conditions of the tissue
